
 產(chǎn)業(yè)資訊
產(chǎn)業(yè)資訊
 藥明康德
藥明康德  2024-10-22
2024-10-22
 112
112
本期看點(diǎn)
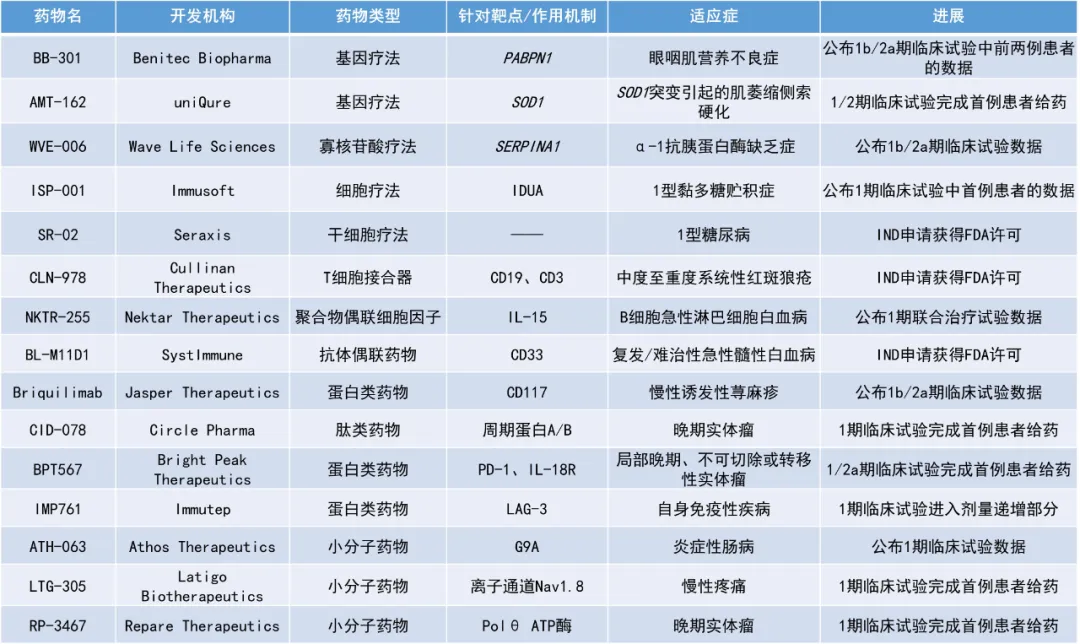
1. 新型IL-15受體激動(dòng)劑NKTR-255聯(lián)用嵌合抗原受體(CAR)-T細(xì)胞療法治療復(fù)發(fā)或難治性B細(xì)胞急性淋巴細(xì)胞白血病(B-ALL)悦级,患者12個(gè)月時(shí)的無(wú)進(jìn)展生存率是單獨(dú)使用CAR-T細(xì)胞療法歷史對(duì)照組的兩倍(67%對(duì)比38%)。
2. 單抗療法briquilimab在針對(duì)慢性誘發(fā)性蕁麻疹(CIndU)患者開(kāi)展的1b/2a期研究中表現(xiàn)亮眼辫航,93%的患者在接受治療后6周達(dá)成緩解斑渠。
3. 首個(gè)進(jìn)入人體臨床試驗(yàn)的工程化B細(xì)胞療法ISP-001的初步結(jié)果積極,首位接受治療的1型黏多糖貯積癥(MPS I)患者已停止使用標(biāo)準(zhǔn)療法漓惕。
藥明康德內(nèi)容團(tuán)隊(duì)整理
NKTR-255:公布1期聯(lián)合治療試驗(yàn)數(shù)據(jù)
Nektar Therapeutics公司公布了一項(xiàng)1期研究的首個(gè)臨床數(shù)據(jù)榕诬,該研究評(píng)估了其新型IL-15受體激動(dòng)劑NKTR-255聯(lián)用靶向CD19和CD22的自體雙特異性CAR-T細(xì)胞療法治療復(fù)發(fā)或難治性B細(xì)胞急性淋巴細(xì)胞白血病患者的效果。NKTR-255是一種聚合物偶聯(lián)人IL-15每贮,它能保持與IL-15受體α亞基的結(jié)合親和力菜犀,并降低清除率,從而提供持續(xù)的藥效學(xué)作用榔汤。NKTR-255具有增強(qiáng)免疫治療效果的潛力丸匀。
此次公布的結(jié)果顯示,NKTR-255聯(lián)用CAR-T細(xì)胞療法治療復(fù)發(fā)或難治性B-ALL患者的療效良好危融。9名患者中有8名獲得了完全緩解(CR)畏铆,伴有或不伴有血液學(xué)恢復(fù),所有患者均未檢測(cè)到微小殘留布辍(MRD)辞居。此外,與僅接受相同CAR-T細(xì)胞療法的歷史對(duì)照組相比蛋勺,NKTR-255聯(lián)用CAR-T細(xì)胞療法組患者的12個(gè)月無(wú)復(fù)發(fā)生存率更高(67%對(duì)比38%)瓦灶。歷史對(duì)照組患者的中位無(wú)復(fù)發(fā)生存期(RFS)為3.9個(gè)月,聯(lián)合治療組患者接受隨訪超過(guò)14個(gè)月抱完,中位RFS尚未達(dá)到贼陶。安全性方面,未觀察到與NKTR-255相關(guān)的劑量限制性毒性巧娱。聯(lián)合治療組中觀察到的毒性與單獨(dú)使用CAR-T細(xì)胞治療后觀察到的毒性相似碉怔。
Briquilimab:公布1b/2a期臨床試驗(yàn)數(shù)據(jù)
Jasper Therapeutics公司公布了其候選單抗briquilimab用于治療慢性誘發(fā)性蕁麻疹的1b/2a期研究SPOTLIGHT的初步數(shù)據(jù)。Briquilimab是一種非糖基化單克隆抗體禁添,可通過(guò)靶向細(xì)胞表面受體c-Kit(也稱為CD117)以避免與干細(xì)胞因子結(jié)合撮胧,從而阻斷肥大細(xì)胞中的關(guān)鍵生存信號(hào)庸伏,造成細(xì)胞凋亡與耗竭,從而消除肥大細(xì)胞驅(qū)動(dòng)疾参蕖(如慢性蕁麻疹)中炎癥反應(yīng)的潛在來(lái)源技乡。
此次公布的結(jié)果顯示,CIndU患者在接受briquilimab皮下注射后1周便觀察到CR碎痘,且93%的患者在接受治療后6周達(dá)成緩解技碍。120毫克劑量組中,12名患者中有10名(83%)達(dá)到了CR创靴,1名達(dá)成部分緩解(PR)朱鹤。40毫克組中,1名患者達(dá)成CR秫痪,2名患者達(dá)成PR。該公司已獲得監(jiān)管批準(zhǔn)在SPOTLIGHT研究中招募患者接受更高劑量藥物的治療捅没。
ISP-001:公布1期臨床試驗(yàn)中首例患者的數(shù)據(jù)
Immusoft公司公布了首位接受其工程化B細(xì)胞療法ISP-001治療的1型黏多糖貯積癥患者的積極結(jié)果叭舰。根據(jù)新聞稿,ISP-001是首個(gè)進(jìn)入人體臨床試驗(yàn)的工程化B細(xì)胞療法舀黄。該療法使用非病毒載體將表達(dá)α-L-艾杜糖苷酶的轉(zhuǎn)基因引入B細(xì)胞中忱当,能夠讓經(jīng)過(guò)改造的B細(xì)胞長(zhǎng)期大量生產(chǎn)治療性蛋白,從而改善患者癥狀治专。
此次公布的臨床試驗(yàn)結(jié)果顯示卖陵,首位接受ISP-001治療的患者在接受治療3-8個(gè)月后,尿液中的多糖疾病生物標(biāo)志物水平降低到正常范圍內(nèi)张峰。這名患者腦脊液中的硫酸乙酰肝素(HS)水平在接受治療6個(gè)月時(shí)降低25%泪蔫。這是FDA認(rèn)可的一個(gè)與MPS I疾病活動(dòng)相關(guān)的替代終點(diǎn)。生物標(biāo)志物之外喘批,這名患者的6分鐘行走檢測(cè)表現(xiàn)提高30%撩荣,并且肩部活動(dòng)范圍在6個(gè)月時(shí)得到改善。關(guān)節(jié)僵硬是MPS I的癥狀之一饶深。在接受治療8個(gè)月后餐曹,該患者停止使用標(biāo)準(zhǔn)酶替代療法(ERT)。隨后的一個(gè)月里敌厘,患者的6分鐘行走檢測(cè)表現(xiàn)仍然獲得改善台猴,不過(guò)幅度比6個(gè)月時(shí)有所下降,肩部活動(dòng)范圍的改善得到維持俱两。安全性方面饱狂,ISP-001的耐受性良好。截至2024年9月19日舶酒,尚未報(bào)告任何不良事件疏悯。
WVE-006:公布1b/2a期臨床試驗(yàn)數(shù)據(jù)
Wave Life Sciences公司公布了其在研RNA編輯候選療法WVE-006用于治療α-1抗胰蛋白酶缺乏癥(AATD)的1b/2a期臨床試驗(yàn)RestorAATion-2的積極結(jié)果灸尾。AATD是一種可影響肺和肝臟功能的遺傳疾病,患者除了缺乏具有正常功能的α-1抗胰蛋白酶(AAT)歉冷,其突變型AAT蛋白的積累也會(huì)損害肝臟和肺部汗势。WVE-006是一款通過(guò)PN化學(xué)修飾和GalNAc偶聯(lián),以皮下注射方式給藥的潛在“first-in-class”RNA編輯寡核苷酸療法乳后。WVE-006的設(shè)計(jì)旨在修復(fù)SERPINA1等位基因編碼mRNA中的單堿基突變锁熟,從而恢復(fù)血液中的功能性野生型AAT蛋白,同時(shí)減少異常AAT蛋白的存在确奄,以潛在治療AATD相關(guān)的肺病和/或肝病麸媒。
此次公布的結(jié)果顯示,WVE-006能夠成功編輯AATD患者的基因闪铸。基線時(shí)未能檢測(cè)到患者體內(nèi)的AAT蛋白胚览,而在試驗(yàn)第15天時(shí),患者的平均總AAT蛋白水平達(dá)到10.8微摩爾需五,達(dá)到了監(jiān)管機(jī)構(gòu)批準(zhǔn)AAT增強(qiáng)療法的標(biāo)準(zhǔn)鹉动。此外,早在試驗(yàn)第3天宏邮,患者的總AAT和M-AAT蛋白水平即較基線增加泽示,并持續(xù)到第57天。根據(jù)新聞稿蜜氨,WVE-006是首個(gè)進(jìn)入臨床開(kāi)發(fā)的RNA編輯療法械筛,該試驗(yàn)結(jié)果是RNA編輯療法在人體當(dāng)中完成的首次機(jī)制驗(yàn)證。
BB-301:公布1b/2a期臨床試驗(yàn)中前兩例患者的數(shù)據(jù)
Benitec Biopharma公司公布了其開(kāi)發(fā)用于治療眼咽肌營(yíng)養(yǎng)不良(OPMD)的沉默和取代基因療法BB-301在早期臨床試驗(yàn)中的前兩例患者的積極數(shù)據(jù)飒炎。該療法利用一種新型的埋哟、經(jīng)過(guò)改良的AAV9載體,表達(dá)一種獨(dú)特的郎汪、單一的雙功能構(gòu)建體定欧,從而能夠促進(jìn)密碼子優(yōu)化的PABPN1基因和兩個(gè)針對(duì)PABPN1突變體siRNA的共同表達(dá)。這兩個(gè)siRNA被塑造成微RNA骨架怒竿,以抑制有缺陷的PABPN1突變體的表達(dá)砍鸠,同時(shí)允許表達(dá)經(jīng)過(guò)密碼子優(yōu)化的PABPN1基因,用PABPN1蛋白的功能性版本取代突變體耕驰。
兩名患者接受了最低劑量的BB-301治療京佃,未報(bào)告值得注意的不良事件。此次公布的結(jié)果顯示言丧,1號(hào)患者和2號(hào)患者分別在接受BB-301治療9個(gè)月和6個(gè)月后崔狂,吞咽功能得到了持久且有臨床意義的改善。其中,在基線時(shí)癥狀更輕的2號(hào)患者的悉尼吞咽問(wèn)卷評(píng)分已達(dá)到了臨床定義的正常水平茶链。新聞稿指出臊瞬,這是使用新型基因療法成功改善OPMD患者吞咽功能的首次報(bào)道。
SR-02:IND申請(qǐng)獲得FDA許可
Seraxis公司宣布颓之,其新型胰島替代療法SR-02的IND申請(qǐng)已獲得FDA許可银景,可開(kāi)展1/2期臨床研究。SR-02由同種異體胰腺內(nèi)分泌細(xì)胞簇組成鹤梳,當(dāng)被植入網(wǎng)膜時(shí)诽粪,可在患者自身的胰腺外形成功能性內(nèi)分泌胰腺。SR-02能夠以臨床規(guī)模生產(chǎn)岁九,由健康供體胰腺組織進(jìn)行重編程后產(chǎn)生的專有干細(xì)胞系制成君铁。新聞稿指出,SR-02作為一種有潛力功能性治愈需要胰島素的糖尿病患者的療法据块,是FDA許可的首個(gè)可用于人體試驗(yàn)的重編程干細(xì)胞衍生的候選胰腺產(chǎn)品码邻。
CLN-978:IND申請(qǐng)獲得FDA許可
Cullinan Therapeutics公司宣布,美國(guó)FDA批準(zhǔn)了其新型另假、高效的CD19 x CD3靶向雙特異性T細(xì)胞接合器CLN-978的IND申請(qǐng)像屋,其針對(duì)中度至重度系統(tǒng)性紅斑狼瘡(SLE)患者的全球1期臨床試驗(yàn)可在美國(guó)開(kāi)展。新聞稿指出浪谴,CLN-978是首個(gè)在自身免疫疾病領(lǐng)域獲得美國(guó)FDA授予IND批準(zhǔn)的在研CD19靶向T細(xì)胞接合器。
CLN-978可在體外和體內(nèi)引發(fā)對(duì)表達(dá)CD19的靶細(xì)胞的定向裂解因苹。CLN-978與CD19的結(jié)合親和力極高苟耻,可有效靶向B細(xì)胞,包括CD19水平極低的B細(xì)胞扶檐。CLN-978的分子量行渍取(65 kDa),含有兩個(gè)單鏈可變片段款筑,一個(gè)與CD19結(jié)合智蝠,另一個(gè)與T細(xì)胞上的CD3結(jié)合,還有一個(gè)與人血清白蛋白結(jié)合的單結(jié)構(gòu)域抗體浩出,可延長(zhǎng)血清半衰期铭梯。CLN-978有望為SLE和類風(fēng)濕性關(guān)節(jié)炎等自身免疫疾病患者提供一種方便、現(xiàn)貨型扶蜻、皮下注射的治療選擇巷同。
參考資料
[1] Immusoft to Announce Positive Phase 1 Data for First Engineered B Cell Therapy in a Clinical Trial. Retrieved October 15, 2024, from https://www.prnewswire.com/news-releases/immusoft-to-announce-positive-phase-1-data-for-first-engineered-b-cell-therapy-in-a-clinical-trial-302255551.html
[2] Seraxis Announces FDA IND Allowance for Clinical Study of SR-02 Replacement Islets for Type 1 Diabetes. Retrieved October 18, 2024, from https://seraxis.com/wp-content/uploads/2024/10/Seraxis-IND-clearance-PR-FINAL-for-release-15Oct2024.pdf
[3] Nektar Announces Publication in Blood of Phase 1 Data for Novel IL-15 Agonist NKTR-255 in Combination with Autologous CD19-22 CAR-T Cell Therapy in Patients with B-cell Acute Lymphoblastic Leukemia. Retrieved October 18, 2024, from https://ir.nektar.com/news-releases/news-release-details/nektar-announces-publication-blood-phase-1-data-novel-il-15
[4] Jasper Therapeutics Reports Positive Data from SPOTLIGHT Study of Briquilimab in Chronic Inducible Urticaria. Retrieved October 14, 2024 from https://www.globenewswire.com/news-release/2024/10/14/2962446/0/en/Jasper-Therapeutics-Reports-Positive-Data-from-SPOTLIGHT-Study-of-Briquilimab-in-Chronic-Inducible-Urticaria.html
[5] Wave Life Sciences Announces First-Ever Therapeutic RNA Editing in Humans Achieved in RestorAATion-2 Trial of WVE-006 in Alpha-1 Antitrypsin Deficiency. Retrieved October 16, 2024 from https://www.globenewswire.com/news-release/2024/10/16/2964053/0/en/Wave-Life-Sciences-Announces-First-Ever-Therapeutic-RNA-Editing-in-Humans-Achieved-in-RestorAATion-2-Trial-of-WVE-006-in-Alpha-1-Antitrypsin-Deficiency.html
[6] Benitec Biopharma Reports Positive Data from Two Subjects Treated with Low-Dose BB-301 in Phase 1b/2a Study Presented at 29th Annual Congress of the World Muscle Society. Retrieved October 18, 2024, from https://www.globenewswire.com/news-release/2024/10/12/2962249/0/en/Benitec-Biopharma-Reports-Positive-Data-from-Two-Subjects-Treated-with-Low-Dose-BB-301-in-Phase-1b-2a-Study-Presented-at-29th-Annual-Congress-of-the-World-Muscle-Society.html
[7] Cullinan Therapeutics Receives U.S. FDA Clearance of Investigational New Drug Application for CLN-978 Administered Subcutaneously in Patients with Moderate to Severe Systemic Lupus Erythematosus. Retrieved October 18, 2024, from https://investors.cullinantherapeutics.com/news-releases/news-release-details/cullinan-therapeutics-receives-us-fda-clearance-investigational
[8] SystImmune, Inc. Announces FDA Clearance of IND Application for BL-M11D1 in Relapsed/Refractory Acute Myeloid Leukemia. Retrieved October 18, 2024, from https://www.prnewswire.com/news-releases/systimmune-inc-announces-fda-clearance-of-ind-application-for-bl-m11d1-in-relapsedrefractory-acute-myeloid-leukemia-302275098.html
[9] Circle Pharma announces first patients dosed in Phase 1 clinical trial of first-in-class oral Cyclin A/B RxL inhibitor CID-078 for advanced solid tumors. Retrieved October 18, 2024, from https://circlepharma.com/circle-pharma-announces-first-patients-dosed-in-phase-1-clinical-trial-of-first-in-class-oral-cyclin-a-b-rxl-inhibitor-brcid-078-for-advanced-solid-tumors
[10] Immutep Announces First-in-Human Phase I Study of IMP761 Progresses to Dose Escalation Portion of Trial. Retrieved October 18, 2024, from https://www.globenewswire.com/news-release/2024/10/17/2964816/0/en/Immutep-Announces-First-in-Human-Phase-I-Study-of-IMP761-Progresses-to-Dose-Escalation-Portion-of-Trial.html
[11] Bright Peak Therapeutics Announces Dosing of First Patient in Phase 1/2a Clinical Trial of BPT567, a First-in-Class Bifunctional PD1-IL18 Immunoconjugate. Retrieved October 18, 2024, from https://brightpeaktx.com/bright-peak-therapeutics-announces-dosing-of-first-patient-in-phase-1-2a-clinical-trial-of-bpt567-a-first-in-class-bifunctional-pd1-il18-immunoconjugate/
[12] Athos Announces Positive Topline Phase 1 Data for its AI-Generated, Novel, Oral G9A Inhibitor ATH-063, Demonstrating Selective Expansion and Activation of Potent Anti-Inflammatory Regulatory T Cells. Retrieved October 18, 2024, from https://athostx.com/news-posts/athos-announces-positive-topline-phase-1-data-for-its-ai-generated-novel-oral-g9a-inhibitor-ath-063-demonstrating-selective-expansion-and-activation-of-potent-anti-inflammatory-regulatory-t-cells/
[13] Latigo Biotherapeutics Doses First Participant in Phase 1 Clinical Trial of LTG-305 for Non-Opioid Treatment of Pain. Retrieved October 18, 2024, from https://www.prnewswire.com/news-releases/latigo-biotherapeutics-doses-first-participant-in-phase-1-clinical-trial-of-ltg-305-for-non-opioid-treatment-of-pain-302276944.html
[14] IgGenix Announces First Patient Dosed in Phase 1 Clinical Trial "ACCELERATE Peanut" Evaluating IGNX001 in Peanut Allergy. Retrieved October 18, 2024, from https://www.prnewswire.com/news-releases/iggenix-announces-first-patient-dosed-in-phase-1-clinical-trial-accelerate-peanut-evaluating-ignx001-in-peanut-allergy-302277256.html
[15] Repare Therapeutics Doses First Patient in Phase 1 Clinical Trial of RP-3467, a Polθ ATPase Inhibitor. Retrieved October 18, 2024, from https://ir.reparerx.com/news-releases/news-release-details/repare-therapeutics-doses-first-patient-phase-1-clinical-trial-2
[16] uniQure Announces Dosing of First Patient in Phase I/II Clinical Trial of AMT-162 for the Treatment of SOD1-ALS. Retrieved October 18, 2024, from https://www.globenewswire.com/news-release/2024/10/15/2963063/0/en/uniQure-Announces-Dosing-of-First-Patient-in-Phase-I-II-Clinical-Trial-of-AMT-162-for-the-Treatment-of-SOD1-ALS.html#:~:text=LEXINGTON%2C%20Mass.%20and%20AMSTERDAM%2C%20Oct.%2015%2C%202024%20%28GLOBE,a%20rare%2C%20inherited%20and%20progressive%20motor%20neuron%20disease.

 產(chǎn)業(yè)資訊
產(chǎn)業(yè)資訊
 生輝
生輝  2024-11-25
2024-11-25
 7
7

 產(chǎn)業(yè)資訊
產(chǎn)業(yè)資訊
 Medaverse
Medaverse  2024-11-25
2024-11-25
 7
7

 產(chǎn)業(yè)資訊
產(chǎn)業(yè)資訊
 醫(yī)藥觀瀾
醫(yī)藥觀瀾  2024-11-25
2024-11-25
 6
6
 熱門(mén)資訊
熱門(mén)資訊 熱點(diǎn)標(biāo)簽
熱點(diǎn)標(biāo)簽